Central Drug Standard Control Organisation (CDSCO), Plastic waste management, and Legal metrology departments regulate cosmetic manufacturing regulations in India.
Under section 3(aaa) of the Drug and Cosmetic Act,1940, cosmetics are any article whose intended purpose includes rubbing, pouring, sprinkling, spraying, or introducing into the human body or any part or applied to increase beauty, attractiveness, and appearance alteration.
No one can manufacture cosmetics if they don’t comply with the specification mentioned in the Ninth schedule or any other standards to maintain safety and quality.
Cosmetics manufacturing license in India is the responsibility of the state license authority license authorised by CDSCO across all the States and Union territories.
Any company that wants to manufacture cosmetics in India has to obtain a manufacturing license for cosmetics from SLA.
APPLICATION FORM | PERMISSION FORM | FEES | LICENSING AUTHORITY |
Form Cos – 5: Application to manufacture cosmetic | Form Cos – 8: Permission to manufacture cosmetics | Rs. 10,000/- (Ten items of each category are free) |
CDSCO |
Form Cos – 6: Application for loan license to manufacture cosmetics. | Form Cos – 9: Permission for loan license to manufacture cosmetics. | Rs. 2500 with inspection fees of 1k for each inspection. |
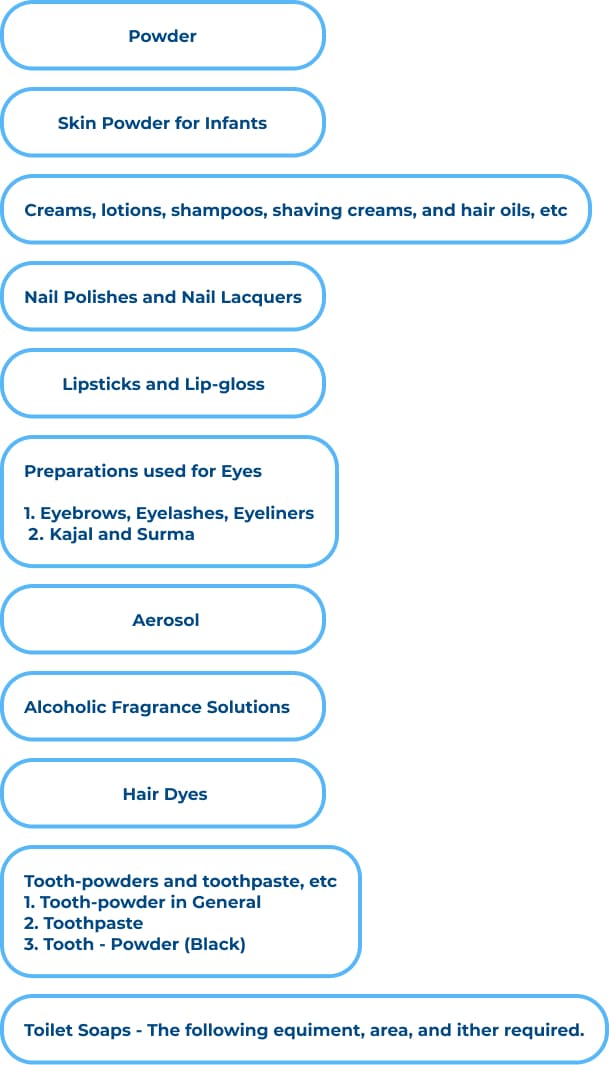
List of cosmetics categories for grant of license to manufacture for sale of cosmetics in the country:

DOCUMENTS REQUIRED BY DIFFERENT DEPARTMENTS | ||
CDSCO | LPMC | PWM |
Form Cos –5; Adhar card number with valid mobile number (mandatory) | Manufacturer’s names and address. | Copy of valid Consent to Operate. |
Fees challan; Cover letter | Name and address of packer (if packed somewhere else). | Copy of registration certificate issued by the Ministry of Micro, Small, and Medium Enterprises |
Ownership of the cosmetics brand. | Generic name of cosmetic.
| Manufacturing process flowchart & Description. |
Documents of the firm constitution, such as the article of association | Name and number of cosmetic products (if packed more than one). | Specifications of Pollution Control devices. |
Declaration of partners and the list with the names of Directors, Partners, and Trustees with their complete addresses | Net weight | Images of the unit/production site. |
Power of Attorney on behalf of the company to authorized agents to submit applications | Month and year of manufacturing. | Specification and related documents of plastic being ordered or supplied with details like the name of the industry, plastic packing type, quantity, etc. |
Approved layout plan for manufacturing site with the section-wise layout of the site and machines. | MRP
| A copy of the integrated manufacturer’s responsibility approved by the Urban Development department |
Possession of documents such as ownership. Registry, lease, rent papers. | Name and address to file a complaint.
| An EPR compliance report in case of registration renewal. |
Master file of the site. | Documents of proof of cosmetic business |
|
List of plant, machinery section wise, and safety equipment | Proof of cosmetic manufacturing site |
|
Technical staff’s full particulars. | Detail of material used in packaging the cosmetics |
|
Declaration of manufacturing analytical chemist | Label pasted on the cosmetic |
|
List of cosmetic products with their composition formula and manner of labelling. | List of all cosmetic products being manufactured. |
|
Manufacturing process details, flow chart of the process. |
|
|
Details of the water system with water testing report. |
|
|
Registration from the district industries centre. |
|
|
Consent for manufacturing site from state pollution control. |
|
|
NOC from the fire safety office |
|
|
Approval from the government for testing. |
|
|
Bonus Points:
CDSCO | LPMC | PWM |
Inspection of manufacturing site: | Director nomination: | Annexure I: |
The State Licensing Authority conducts a site inspection of the entire premises before licensing the manufacturer. | The company must inform the concerned controller or their authorized officer by giving a duplicate notice (format present in schedule no. thirteen). | As per Annexure I, there are three alternative paths to implement the EPR Plan for the PWM in the states/UT they are planning to set up their manufacturing site. |
Ø Labeling requirements as per Drugs and Cosmetics Rules, 1945 for cosmetics products are: | It consists of the name and address of the director with its writing consent, whom the company has nominated as per section 49 sub-section (2). | a) With their distribution channel. |
The outer label shall have a clear visible ingredient list used in cosmetic product manufacturing. | The director would be responsible for running the company or manufacturing unit. | b) With the direct participation of the municipality. |
The batch number starting with B and the manufacturing batch starting with M shall be present on the label. |
| c) With the waste management agency (WMA).
|
The product names shall be visible on the outer and inner labels. |
|
|
If the product is inside the container or in a small size, then manufacturing places and zip codes are acceptable. |
|
|
Directions to use and warning shall be present on the inner label. |
|
|

- Within 45 days, SLA can reject the application if it is incomplete.
- After the company gets the license, it must upload it to the CDSCO website.
- Within 30 days of giving the license, the SL inspects the site to verify the information.
Conclusion: Due to tight cosmetics manufacturing regulations in India, the manufacturers are advised first to understand the requirement and procedures for the license. To continue the smooth sale, distribution, and cosmetics manufacturing, the manufacturers must comply with the prescribed regulations to save themselves from license cancellation or penalty.
Avoid These 10 Common Mistakes When Applying for CDSCO Licenses: Download Now
Ensure a successful CDSCO license application by avoiding these common mistakes. Download now to understand the pitfalls and improve your chances of approval.







