The import of cosmetics is regulated by the Central Drugs Standard Control Organization (CDSCO), Ministry of Health and Family Welfare under the Drugs and Cosmetics Act 1940, and the new Cosmetic Rules 2020. It governs all procedures such as registration, inspection, and licensing requirements to import cosmetics.
As per the Drugs and Cosmetics Act, 1940, Cosmetics is “any article that can be rubbed, poured, sprinkled, sprayed, applied or introduced to any part of the human body to increase the beauty, appearance or attractiveness or any part that consumers can use in cosmetic.
Under rule 12, the government of India has made it compulsory to have a registration certificate to import cosmetics in India.
An import registration certificate means issuing a certificate by the Central Licensing Authority under rule 13 to register cosmetics manufactured to import in India.
India imports their cosmetic products from countries like Canada, Italy, the US, China, and Korea, and in the coming years, the import percentages are expected to increase.
Thus, complying with cosmetic products with the Drug and Cosmetic Act and rules helps the importers not be stuck in legal activities. Here are the following points everyone importing cosmetics products in India should remember.
The Rules ban the cosmetic import in the following cases:
- No one shall import, manufacture, sell or distribute cosmetics banned in the origin country under the same or any name except for the examination, test, or analysis.
- If the “Use Before or Use by date” is less than six from the import date, it shall not be imported.
- Anyone shall not import cosmetics containing Hexachlorophene.
- Cosmetics tested on animals after 12th November 2014 shall not be imported.
The import license is a prerequisite to importing those cosmetic products not manufactured in India. The importers can use Form -1 and Form 2, issued by the central licensing authority. A fee of $250 or equivalent to the Indian rupee is paid on the SUGAM portal through challan.
- Cos Form 1- application form to get the import license.
- Cos Form 2- Permission to import cosmetics to India.
Application Form | Approval Form | Fees | Licensing authority | Validity | Penalty |
Form Cos -1 | Form Cos -2 | $2000+ $50 for each variant | CDSCO | Three years | Under section 10 A, a person can get imprisonment for three years, a five thousand fine, or both. |
Importers can be anyone who:
- Is a manufacturer with a registered office in India.
- Is an authorized agent of the manufacturer.
- Is a subsidiary manufacturer.
- Any importer.
- An authorized agent is a person or organization in India approved by the manufacturer. They will be responsible for running business activities in India on behalf of the manufacturer, checking compliance with the Drug and Cosmetics Act.
- Subsidiary means any manufacturer-owned unit in India.
- Any other importer means any person or unit planning to import cosmetic products.

DOCUMENTS REQUIRED BY DIFFERENT DEPARTMENTS | ||
CDSCO | LPMC | PWM |
Cover letter -The importer should mention the purpose of importing cosmetics and earlier issued import registration. | Manufacturers’ names and addresses. | Copy of valid Consent to Operate. |
Cos Form 1: This form is submitted after the importer pays the fees online. | Name and address of packer (if packed somewhere else). | Copy of registration certificate issued by the Ministry of Micro, Small, and Medium Enterprises |
Challan: Contains information about the applicant as well as payment made. | Generic name of cosmetic.
| Manufacturing process flowchart & Description. |
Power of attorney: Consists of information about the manufacturer’s contract and importer list of products to be imported with their category and pack size | Name and number of cosmetic products (if packed more than one). | Specifications of Pollution Control devices. |
Schedule D: information on the manufacturer and their facilities. | Net weight | Images of the unit/production site. |
Label: Contains information such as name, address, country of manufacturing, batch no, etc. | Month and year of manufacturing. | Specification and related documents of plastic being ordered or supplied with details like the name of the industry, plastic packing type, quantity, etc. |
Free sale certificate: A free sale certificate, marketing authorization letter, and manufacturing license for each variant. | MRP
| A copy of the integrated manufacturer’s responsibility approved by the Urban development department |
Product specifications.
| Name and address to file a complaint.
| An EPR compliance report is needed in case of registration renewal. |
Pack insert | Documents of proof of cosmetic business. |
|
Animal testing and heavy metal declaration. | Proof of cosmetic manufacturing site. |
|
List of nations where the product has been authorized to import or market. | Detail of material used in packaging the cosmetics. |
|
| Label pasted on the cosmetic |
|
| List of all cosmetic products being manufactured. |
|
Bonus points
CDSCO | LPMC | PWM |
| Director nomination: | Annexure I: |
After getting the registration certificate, if there are any changes in specification, ingredients, variants, etc., the CLA needs to be informed at least 30 days before the import. | The company must inform the concerned controller or their authorized officer by giving a duplicate notice (format present in schedule no. thirteen). | As per Annexure I, there are three alternative paths to implement the EPR Plan for the PWM in the states/UT they are planning to set up their manufacturing site. |
The applicants must provide the translated version if any documents are in any foreign language. | It consists of the name and address of the director with its writing consent, whom the company has nominated as per section 49 sub-section (2). | a) With their distribution channel. |
An affidavit shall be present if there is no provision for a manufacturing license for cosmetics in a country. | The director would be responsible for running the company or manufacturing unit. | b) With the direct participation of the municipality. |
Those cosmetics products imported for R & D purpose registration certificate is not required. |
| c) With the waste management agency (WMA). |
Fresh/ New registration cases: · Any change in name, constitution, importer, authorized agent, name, or the constitution of authorized agent. · In case of acquisition or merger. |
|
|
No fresh/new registration cases: · Method test change. · Composition change · Updates in packs and labels . Minor manufacturing changes not affecting the final product. |
|
|
Steps to get an import license for cosmetic products:
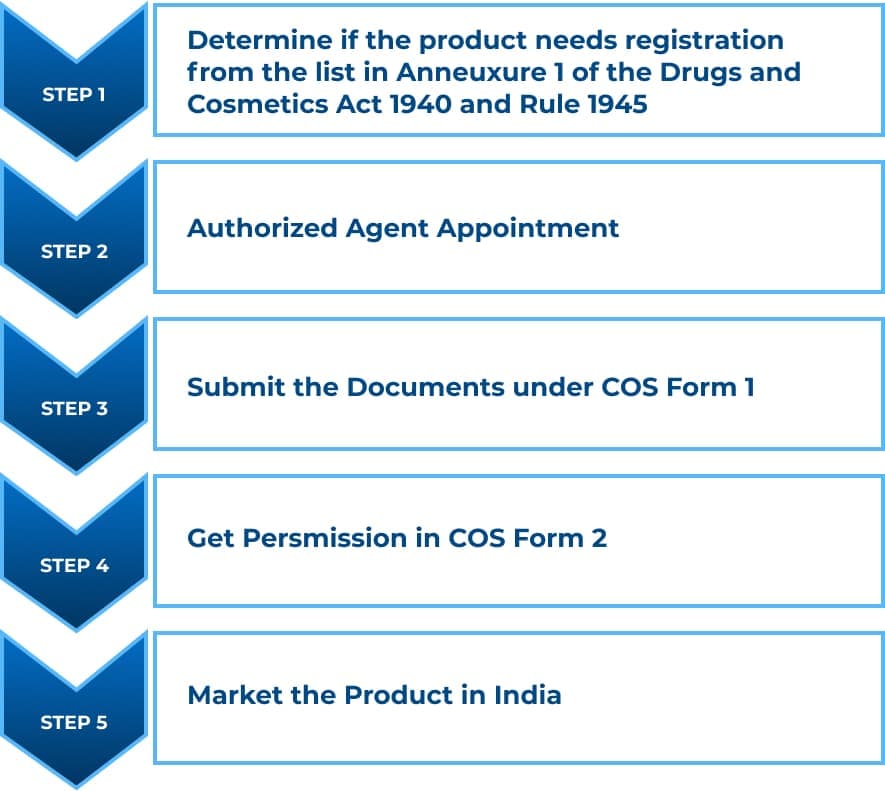
Conclusion: Most of the time, an application gets rejected, or a product does not get the import license due to discrepancies in the documents submitted or steps that need to be completed. When a product does not comply with the regulations of a country, selling it becomes problematic. Thus, before marketing any product, it is advisable to have full knowledge of the rules and documents.
10 Common Mistakes Importers Make When Applying for CDSCO Licenses: Download and Learn
Learn from the experiences of other importers and avoid these common mistakes when applying for CDSCO licenses. Download now to optimize your application process.







