India is one of the world’s largest producers of medical and in-vitro medical devices, with thousands of devices manufactured every year. The manufacturing and sale of these devices are governed by the Central Drugs Standard Control Organization (CDSCO), which regulates their safety, efficacy, and quality.
The CDSCO’s main objective is to maintain a balance between the benefits and risks of medical devices, ensuring that they meet the highest safety and quality standards. To achieve this, the CDSCO has established a comprehensive regulatory framework that includes licensing, inspection, and post-market surveillance.
In 2022, the Indian government announced the National Medical Devices Policy, which aims to reduce the country’s dependence on imports and promote domestic manufacturing. Under this policy, the government will provide incentives and support to medical device manufacturers, such as expedited licensing and regulatory approvals, tax incentives, and funding for research and development.
The policy is expected to stimulate growth in the medical device industry and create new opportunities for both local and foreign manufacturers. As the Indian medical device market continues to expand, it is essential for companies to stay abreast of the latest regulations and requirements to ensure compliance and maintain a competitive edge.
Definition of Medical Device and In- vitro as per Medical Device Rule,2017.
DEFINITION
Medical Devices
1. All those substances used for in-vitro diagnosis or surgical purposes, such as dressings, bandages, sutures, ligatures, blood, and its component collection bags with or without coagulant under sub-clause (i).
2. Substances such as mechanical contraceptives (condoms, intrauterine devices, tubal rings), disinfectants, and insecticides, notified in the Official Gazette under sub-clause (ii),
3.Devices are notified from time to time under sub-clause (iv) of clause (b) of section 3 of the Act.
In-Vitro Medical Devices
As per sub-clause (A) of clause (ZB), medical devices used in vitro diagnosis not approved by the CLA for importation and are tested to establish their performance for relevant analysis or other parameters related to it, including details of technology and the procedure required;
The Medical Devices/ In-Vitro Diagnostic devices are classified as follows:
Class | Medical Device | In-Vitro Diagnostic |
A – Low risk | ✓ | ✓ |
B – Low Moderate Risk | ✓ | ✓ |
C – Moderate Risk | ✓ | ✓ |
D – High Risk | ✓ | ✓ |
To Import Medical Devices/ In Vitro Diagnostics Medical devices in India, the below-listed Application Forms need to file with Licensing Authority.
Form No. | Description | Devices and Class | Fees | Licensing Authority |
MD Form – 14: | Application to import medical devices | Only medical devices and classes A, B, C & D. | For class A: $1000 & $500 per product. For class B: $2000 & $500 per product. For class C & D: $3000 & & $1500 per product. |
CDSCO |
MD Form -15 (Approval) | Permission to import medical devices. | |||
MD Form – 28 | Application to import In-Vitro medical devices | Only In-Vitro medical devices and classes A, B, C & D. | For classes A & B: $1000 & $10 per product. For class C & D: $3000 & $500 per product. | |
MD Form – 29: | Permission to import In-Vitro medical devices. |
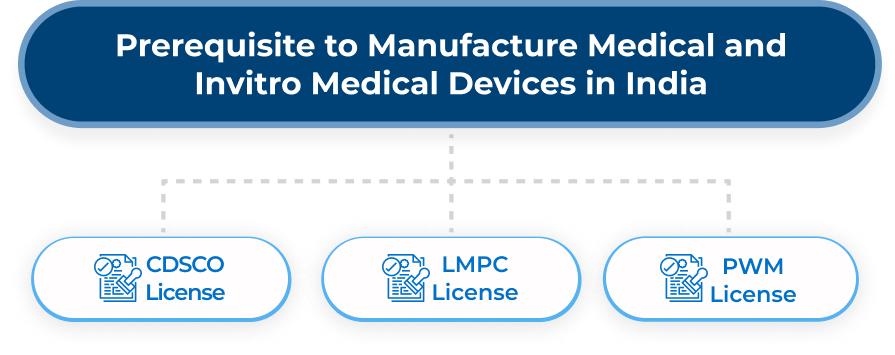
CDSCO – The Central Drugs Standard Control Organization (CDSCO) is responsible for regulating the manufacturing and sale of medical devices in India. Under the control of the Drug Controller General of India (DCGI), the CDSCO follows the Drug and Cosmetic Act of 1940 and rules of 1945.
The CDSCO classifies medical devices into two categories: Notified Devices and Non-notified devices. Notified Devices, numbering 37 in total, require prior permission from the CDSCO before they can be sold in the Indian market. Non-notified devices must be present on the CDSCO portal, and foreign manufacturers who want to sell devices must have an Indian Authorized Agent (IAA).
In addition to CDSCO regulations, the Legal Metrology Packaged Commodity Act of 2009 and rules of 2011 is a mandatory license for the manufacture of medical and in-vitro medical devices in India. The main objective of legal metrology is to ensure firms follow measurement and packaging rules established by the department.
Furthermore, the Plastic Waste Management Rule of 2016 aims to reduce plastic usage and waste. Obtaining a PWM license is essential when setting up a medical and in-vitro medical device manufacturing site to avoid delays in the manufacturing process. Compliance with these regulations is critical for manufacturers to ensure safety, quality, and environmental sustainability.
|
CDSCO |
LPMC |
PWM |
|
|
Medical devices (class A, B, C & D) |
In-Vitro Medical devices (class A, B, C & D) |
For medical and In-Vitro medical devices (class A, B.C & D) |
For medical and In-Vitro medical devices (class A, B.C & D) |
|
Applicants Details |
Cover letter |
Manufacturers’ names and addresses. |
Copy of valid Consent to Operate. |
|
Information of product |
Documents related to regulatory |
Name and address of packer (if packed somewhere else). |
Copy of registration certificate issued by the Ministry of Micro, Small, and Medium Enterprises |
|
Status of regulatory |
Power of Attorney |
Generic name of medical and in-vitro medical devices. |
Manufacturing process flowchart & Description. |
|
Clearance from other countries. |
ISO 13485 Certificate |
Name and number of medical and in-vitro medical devices (if packed more than one). |
Specifications of Pollution Control devices. |
|
Master file |
Fees Challan |
Net weight |
Images of the unit/production site. |
|
The device has a medicinal product. |
Certificates of GMP, CE design, free sale, and batch release certificates. |
Month and year of manufacturing. |
Specification and related documents of plastic being ordered or supplied with details like the name of the industry, plastic packing type, quantity, etc. |
|
Post-market surveillance information |
Conformity Declaration |
MRP |
A copy of the integrated manufacturer’s responsibility approved by the Urban development department |
|
Report of PMS |
Name and address to file a complaint. |
An EPR compliance report in case of registration renewal. |
|
|
Business License / Plant Registration Certificate and Quality certificate by overseas Manufacturer assurance of product’s quality. |
Documents of proof of medical and in-vitro medical devices business. |
||
|
|
|
|
|
|
Medical devices (class A, B, C & D) |
In-Vitro Medical devices (class A, B, C & D) |
For medical and In-Vitro medical devices (class A, B.C & D) |
For medical and In-Vitro medical devices (class A, B.C & D) |
|
Audit Report and test report with details. |
Proof of medical and in-vitro medical devices manufacturing site. |
||
|
Constitutional Details of partners. |
Detail of material used in packaging the medical and in-vitro medical devices. |
||
|
The authorized agent must have a valid wholesale or manufacturer license. |
Label pasted on the medical and in-vitro medical devices. |
||
|
Plan master files |
List of all medical and in-vitro medical devices being manufactured. |
||
|
Director nomination: |
Annexure I: |
||
|
The company must inform the concerned controller or their authorized officer by giving a duplicate notice (format present in schedule no. thirteen). |
As per Annexure I, there are three alternative paths to implement the EPR Plan for the PWM in the states/UT they are planning to set up their manufacturing site. |
||
|
It consists of the name and address of the director with its writing consent, whom the company has nominated as per section 49 sub-section (2). |
a) With their distribution channel. |
||
|
The director would be responsible for running the company or manufacturing unit. |
b) With the direct participation of the municipality. |
||
|
|
|
|
|
|
Medical devices (class A, B, C & D) |
In-Vitro Medical devices (class A, B, C & D) |
For medical and In-Vitro medical devices (class A, B.C & D) |
For medical and In-Vitro medical devices (class A, B.C & D) |
|
c) With the waste management agency (WMA). |
|||
Steps to get a manufacturing license for medical and in-vitro medical devices:
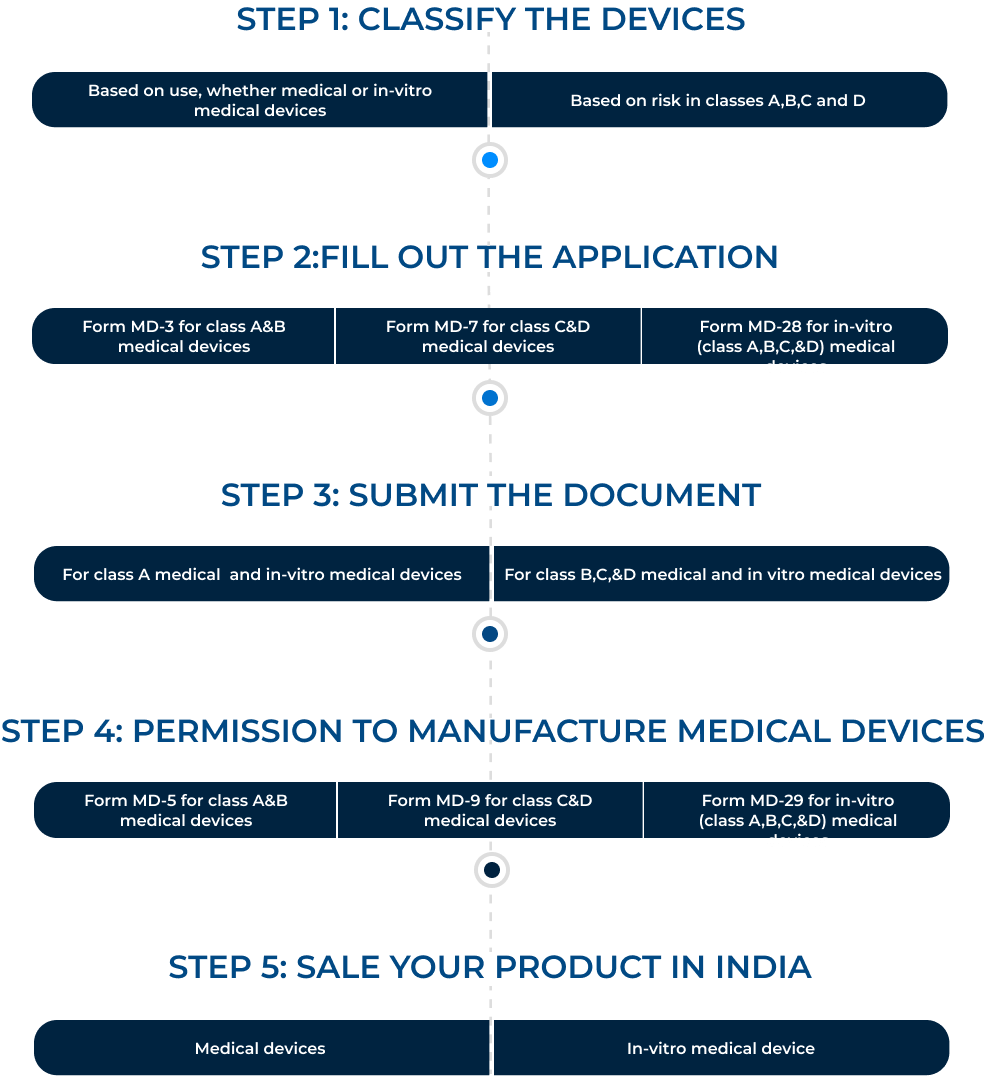
Conclusion: Most individuals believe that obtaining just a CDSCO license is sufficient authorization to establish a production facility for in-vitro and medical products. But they are astonished when they find out that other licenses are necessary for addition to CDSCO.







