Regulatory bodies of India continuously update themselves with the latest technology
Unlock the Secrets to Navigating India’s Healthcare and Personal Care Landscape: Download Your Free Guide to the Top Regulatory Bodies Now
In the past few years, there have been rapid updates from the regulatory bodies of healthcare and personal care on different rules and regulations to make the products safer.
The main objective of regulatory bodies in the healthcare sector is to help deliver products such as medical devices, drugs, and cosmetics with high benefits and no or minimal risks.
The main functions of the regulatory body include
- Corrective action,
- Enforcement of laws and regulations,
- License,
- Packing,
- Post-market reports

S. No | Regulatory body | Full form | Objective/Aim |
1. | CDSCO | Central Drug Standard Control Organization | It is controlled by the DGCI (Drug Controller General of India). Its headquarters is located in Delhi. ⮚ CDSCO works as a national-level regulatory body under the Ministry of health and family welfare. ⮚ CDSCO is responsible for approving licenses to import, manufacture, sell or distribute drugs or medical devices, Cosmetics, and Vaccines, and Conduct Clinical Trials, to name a few, and classifying them. ⮚ CDSCO operates six zonal, seven sub-zonal, thirteen airports, and seven laboratories. |
2. | AYUSH | Ayurveda, Yoga, Naturopathy, Unani, Sidha, and Homeopathy | ⮚ The headquarters of AYUSH is located in New Delhi. The main aim of AYUSH is to upgrade the Indian Systems of Medicine and Homoeopathy colleges. ⮚ It also aims to ensure upscale the research to find effective treatments for the disease and advance the pharmacopeial standard for homoeopathy drugs and medicines. ⮚ AYUSH runs multiple healthcare programs with a high focus on improving rural health. |
3. | BIS | Bureau of Indian Standard | ⮚ It is a national standard body established under the BIS act,2016. The headquarters of BIS is situated in Delhi. ⮚ The main focus of BIS is to harmonise the standards, marking, and quality of all the goods imported, exported, or manufactured in India. ⮚ The BIS works on (i) Framing standards, (ii) International activities, (iii) Certifying the products, (iv) Hallmarking, (v) Labs service, (vi) Giving training, and (vii) Affairs related to consumers.
|
4. | PWM BY CPCB | Plastic Waste Management by Central Pollution Control Board | ⮚ The Plastic waste management rules were notified on 18th march 2016, eliminating the Plastic waste (management and handling) Rules, 2011. Amendments in Rules 2016 were done in 2018, 2021, and 2022. ⮚ The main aim is to manage plastic waste, recycle it, reuse it, collect the waste separately, and bring a sense of responsibility to producers and generators. |
5. | FSSAI | Food safety standard authority of India | ⮚ It was established by the Ministry of Health and Family Welfare of India under the Food Safety and Standards Act, 2006. ⮚ The main aim of the FSSAI is to regulate food safety, quality, and regulations in India. It is the responsibility of the FSSAI to protect and promote public health and supervise the quality and safety of food with the help of rules. ⮚ The FSSAI regulates the food cycle from manufacturing, storage, distribution, sale, and import. |
CDSCO AND ITS DIVISION
There are eight divisions of the CDSCO.
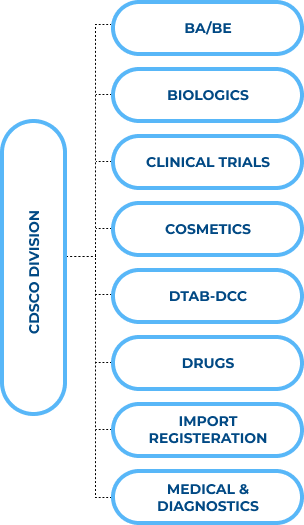
a) Bioavailability (BA): It can be defined as the amount and rate at which the drug given orally is absorbed into the circulatory system and starts working at the site of action.
Bioequivalence (BE): Bioequivalence is the biochemical similarity between two drugs. Those drugs share the same active element and results for the patients. The level and adsorption rate are not much different from the reference product when given the same molar dose.
b) Biologics: Biologics or biological products are medical products extracted from natural sources such as humans, animals, or microbes. Biologics can treat diseases and medical conditions and prevent or diagnose diseases. Examples of Biologics are Vaccines, Hormones, Products made from blood, Cellular and Gene remedies, Monoclonal Antibodies, Allergy shots, Tissues and human cells used for transplantation, and Tests to find potential blood donors against infectious diseases, etc.
c) Clinical Trials: Clinical Trials in India are called clinical research. In clinical research, the researchers test the Drugs, surgeries, and treatments on contestants. As per the National Drugs and Clinical Trials Rule, 2019, from sections 3.7.1 to 3.7.3 of ICMR, all those conducting clinical trials must be publicly documented in the Clinical Trials Registry – India.
d) Cosmetics: Under section 3 (aaa) of the Drugs and Cosmetics Act,1940, the cosmetic is defined as any article which can be rubbed, poured, sprinkled, sprayed, introduced, or applied to any body part of a human. Any article that can be used for cleansing, enhancing beauty, attractiveness, or any product that can be used as a cosmetic.
e) DTAB-DCC: This division organises the meetings of the Drugs Technical Advisory Board (DTAB) and Drugs Consultative Committee (DCC). They mainly share the recommendations on implementing the Drug and Cosmetics Acts and Rules and amending them.
f) Drugs: The rules 122A, 122B,122 DAB, 122 DAC, 122 DD, 122 E, and Appendix I-XII of Schedule Y of Drugs and Cosmetics Rule,1940 contains the information on data required for Approval of clinical trials, import, manufacturing, and marketing of new drugs in the country. As per Rule 122 E of the Drugs and Cosmetics Rule, a new Drug is a drug that has not been used under the prescribed conditions and is considered unsafe and non-effective by the state licensing authority.
g) Import and Registration: The import of Drugs regulated under Chapters III and IV of the Drugs and Cosmetics Act,1940 and 1945, respectively. The registration and license of drugs regulate as per the requirements of Chapters 24 (A)& 24.
h) Medical and Diagnostic Devices- Under the Drugs and Cosmetics Act,1940 and Rule,1945, only notified medical devices are regulated, while diagnostic devices regulate under sub-clause (i) and (iv) of clause (b) of section 3.
Conclusion: India is a developing country continuously updating its rules and regulations. CDSCO is the regulatory body that controls different divisions. The collective aim of the CDSCO and its division is to increase the marketed products’ quality, safety, and efficacy.
Download Checklist of Documents Required for Registration of Medical Devices with CDSCO
These comprehensive set of checklists are designed to help streamline the registration process and ensure that you have all the necessary documentation in place.







