Introduction
The healthcare industry plays a pivotal role in safeguarding public health and well-being. However, the rapid advancement of medical technology and the increasing demand for medical devices have led to a significant challenge – the management of electronic waste (e-waste) generated from these devices. India, a rapidly growing economy with a burgeoning healthcare sector, has recognized the urgency of addressing this issue and has taken decisive steps to regulate e-waste management through the E-Waste (Management) Rules, 2022.
In this comprehensive blog, we will delve into the intricacies of the E-Waste (Management) Rules, 2022, and explore their impact on the medical device industry in India. We will also provide insights into the Extended Producer Responsibility (EPR) framework, the registration process for manufacturers and importers, and the practical steps required for compliance. Additionally, we will shed light on the benefits of adhering to these regulations and the potential consequences of non-compliance.
Understanding the E-Waste (Management) Rules, 2022
On November 2, 2022, the Ministry of Environment, Forests, and Climate Change published the E-Waste (Management) Rules, 2022, which came into effect on April 1, 2023. These regulations aim to establish a comprehensive framework for the effective management of e-waste generated from electrical and electronic equipment (EEE), including medical devices.
The rules apply to every manufacturer, producer, refurbisher, dismantler, and recycler involved in the life cycle of electrical and electronic equipment listed in Schedule I of the
E-Waste (Management) Rules, 2022.
This schedule includes a wide range of medical devices, such as radiotherapy equipment, cardiology equipment, dialysis equipment, pulmonary ventilators, nuclear medicine equipment, laboratory equipment for in-vitro diagnosis, analyzers, imaging equipment (MRI, PET, CT, and ultrasound), fertilization test equipment, and other electric appliances used for medical purposes.
The E-Waste (Management) Rules, 2022, aim to achieve the following objectives:
- Establish an Extended Producer Responsibility (EPR) framework: The rules mandate that manufacturers and producers take full responsibility for the end-of-life management of their products, ensuring safe and environmentally sound disposal.
- Facilitate collection and recycling: Manufacturers and distributors are required to provide designated collection points for consumers to return their e-waste, enabling proper collection, refurbishment, and recycling.
- Regulate recyclers and dismantlers: The government has mandated the registration of recyclers and dismantlers to ensure compliance and proper e-waste management practices.
- Promote awareness and transparency: The rules emphasize the importance of creating awareness about e-waste management and maintaining transparency through reporting and auditing mechanisms.
The Extended Producer Responsibility (EPR) Framework
The E-Waste (Management) Rules, 2022, introduce the Extended Producer Responsibility (EPR) framework, which places the onus of e-waste management on manufacturers and producers. This framework aims to promote a circular economy and ensure the sustainable management of e-waste.
Under the EPR framework, manufacturers and producers are responsible for meeting specific e-waste recycling targets, as outlined in Schedules III and IV of the rules. These targets are calculated based on the quantity of EEE placed in the market and the average lifespan of the product.
Table 1: Medical Device Categories and Codes under E-Waste (Management) Rules, 2022Extended Producer Responsibility (EPR):
Medical Device Category | Code |
Radiotherapy equipment and accessories | MDW1 |
Cardiology equipment and accessories | MDW2 |
Dialysis equipment and accessories | MDW3 |
Pulmonary ventilators and accessories | MDW4 |
Nuclear Medicine Equipment and accessories | MDW5 |
Laboratory equipment for in vitro diagnosis and accessories | MDW6 |
Analyzers and accessories | MDW7 |
Magnetic Resonance Imaging (MRI), Positron Emission Tomography (PET) Scanner, Computed Tomography (CT) Scanner, & Ultrasound Equipment along with accessories | MDW8 |
Fertilization tests equipment and accessories | MDW9 |
Other electric appliances/equipment/kits used for preventing, screening, detecting, monitoring, evaluating, reviewing, examining, investigating, probing, treating illness sickness, disease, disorder, affliction, infection, injury, trauma, abuse or disability including the Mobiles, Tablets or any other device with the features having the potential of sex selection and their accessories | MDW10 |
Table 2: E-Waste Recycling Targets by Year
Sl. No. | Year (Y) | E-Waste Recycling Target (by weight) |
1 | 2023-2024 | 60% of the quantity of an EEE placed in the market in year Y-X, where ‘X’ is the average life of that product |
2 | 2024-2025 | 60% of the quantity of an EEE placed in the market in year Y-X, where ‘X’ is the average life of that product |
3 | 2025-2026 | 70% of the quantity of an EEE placed in the market in year Y-X, where ‘X’ is the average life of that product |
4 | 2026-2027 | 70% of the quantity of an EEE placed in the market in year Y-X, where ‘X’ is the average life of that product |
5 | 2027-2028 | 80% of the quantity of an EEE placed in the market in year Y-X, where ‘X’ is the average life of that product |
6 | 2028-2029 onwards | 80% of the quantity of an EEE placed in the market in year Y-X, where ‘X’ is the average life of that product |
Table 3: E-Waste Recycling Targets for New Producers
Sl. No. | Year | E-Waste Recycling Target (by weight) |
1. | 2023-2024 | 15% of the sales figure for the financial year 2021-22 |
2. | 2024-2025 | 20% of the sales figure for the financial year 2022-23 |
3. | 2025-2026 onwards | 20% of the sales figure of the financial year two years back |
To comply with the EPR obligations, manufacturers and producers can engage third-party organizations, such as Producer Responsibility Organizations (PROs), collection centers, and dealers. However, the ultimate responsibility remains with the producer. Producers must purchase EPR certificates from registered recyclers through an online system and submit these purchases as part of their quarterly returns.
EPR Registration Process:
Entities involved in the life cycle of EEE, including manufacturers, producers, refurbishers, and recyclers, must register on the designated portal under the appropriate category. Conducting business without registration is prohibited, and registered entities must ensure they do not engage with any unregistered manufacturers, producers, recyclers, or refurbishers.
The registration process involves the following steps:
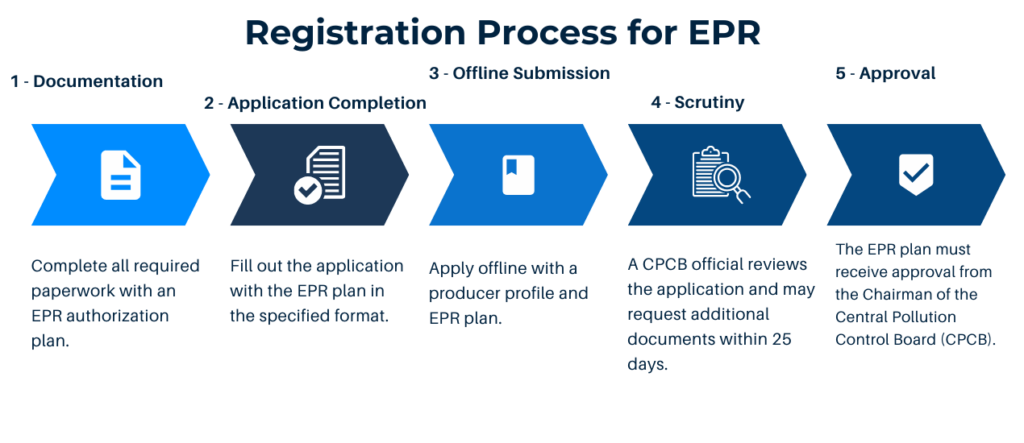
To complete the registration process, manufacturers and importers must provide various documents, including address proof, GST certificate details, company registrar or Import Export Code (IEC) letter, agreement documents with dealers and recyclers, RoHS compliance declaration, technical documents, permissions/licenses, and an estimated EPR budget.
Responsibilities of Producers:
Under the E-Waste (Management) Rules, 2022, manufacturers and producers have specific responsibilities to ensure compliance:
- Registration and Reporting: Both producers must register on the portal and file annual and quarterly returns by the end of the month following the respective quarter or year.
- E-Waste Collection and Recycling: Producers are required to collect and ensure the recycling or disposal of e-waste generated during manufacturing. Producers must obtain and implement EPR targets via the portal, migrating plans from the 2016 rules as needed.
- Awareness and Communication: Producers are responsible for creating awareness about e-waste management through media, publications, advertisements, posters, or other means of communication.
- Storage and Record-keeping: Every manufacturer, producer, refurbisher, and recycler is permitted to store e-waste for up to 180 days (extendable up to 365 days by the CPCB for developing recycling or reuse processes). They must maintain records of the sale, transfer, and storage of e-waste, ensuring these records are available for inspection.
The Role of NKG in E-Waste Management Compliance
Navigating the complex landscape of e-waste management regulations can be challenging for medical device manufacturers and importers. NKG, a leading regulatory compliance consultancy, offers expert guidance and support to help organizations navigate these regulations effectively.
With in-depth knowledge of the CDSCO regulatory environment and extensive experience in the medical device industry, NKG can assist manufacturers and importers in ensuring timely EPR registration, compliance with e-waste management protocols, and seamless import processes.
Conclusion:
The E-Waste (Management) Rules, 2022, mark a significant step towards sustainable management of e-waste in India. Compliance with these rules is crucial for manufacturers, producers, and importers to operate legally and responsibly. The circular from the CPCB provides temporary relief by extending compliance deadlines but underscores the importance of adherence to the rules.
NKG offers expert guidance and support to help manufacturers and importers navigate these regulations effectively. With in-depth knowledge of the CDSCO regulatory environment, NKG can assist in ensuring timely EPR registration, compliance with e-waste management protocols, and seamless import processes. By partnering with NKG Advisory, organizations can minimize legal and financial risks, enhance their brand reputation, and contribute to a sustainable future through responsible e-waste management practices.
As the healthcare industry continues to evolve and advance, it is imperative that medical device manufacturers and importers prioritize environmental sustainability alongside patient care. By embracing the E-Waste (Management) Rules, 2022, and collaborating with regulatory experts like NKG, the medical device industry in India can pave the way for a greener and more sustainable future.