Overview of CDSCO
Unlike the US, Europe, and Canada, India has a regulatory body regulating the registration process of medical devices in India. Central Drug Standard Control Organization (CDSCO) is the regulatory body of India. The main agenda of CDSCO is to market safe and effective devices in the market for the intended population. Therefore, whether a manufacturer or importer, everybody needs to follow the regulations set by the CDSCO to sell and distribute their devices. Without the CDSCO license, selling and distributing the device is impossible, and if found guilty can lead to fines and punishments. On 1st April 2021, CDSCO released a notice to regulate the CT scan, which means any manufacturer or importer dealing with CT scan first now has to get a license. Only then can they continue to sell and distribute.
In this blog, NKG will discuss the registration process for CT scans in India.
What is a CT scan
A computerized tomography (CT) scan is a medical device that combines a series of X-ray images taken from different angles around the body and then uses the computer processor to create cross-sectional images. These images are also referred to as “slices” and are also called tomographic pictures because they have more information than a standard X-ray. Once the computer has several slices, they can be stacked together from 3-D images, making it easy for healthcare professionals to identify and locate the suspect abnormalities in the patients.
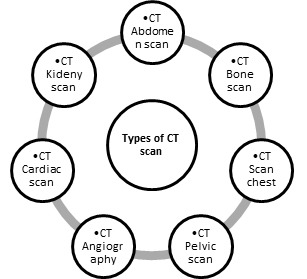
Types of CT scans
Class of CT scan
CT scan belongs to Class C high risk as it involves the use of X-ray, which, if used above the limit, can cause harm to the patients.
Forms required for CT Scan registration
Applicant type | Class | Application | License | Fees |
Manufacturer |
Class C | Form MD-7 | Form MD-9 | For one site, INR 50000, and INR 1000 for a medical device |
Manufacturer (Loan License) | Form MD -8 | Form MD -10 | ||
Importer | Class C | Form MD- 14 | Form MD- 15 | $3000 for one site and $1500 for one medical device unit |
Documents required for CT scan registration
For manufacturers of CT scans:
- Cover letter
- Fees
- Constitution details of the manufacturing firm.
- The Establishment /Site Ownership/Tenancy Agreements.
- Duly notarized quality certificate for manufacturing site(s), if any;
- Copy of the Certificate supporting the quality management system (ISO: 13485), if any;
- Quality management system as per the medical device rule,2017.
- Reference for predicate device,
- Plant master file
- Device master file
- Test license (for the domestic manufacturer)
- Undertaking that the manufacturing site complies with the quality management system.
For the import license authorized agent needs to submit the following documents:
- Cover letter
- Power of attorney
- Wholesale license
- Free sale certificate from the country of origin.
- Free sale certificate from any country, namely USA, Australia, Canada, Japan, UK, and European Union countries.
- Inspection or audit report
- Certificates (ISO, full quality certificate, CE design certificate, Declaration of conformity)
- Plant master file (as per appendix I of MDR, 2017)
- Device master file (as per appendix II of MDR, 2017)
- Label and IFU
- Establishment certificate
- Constitution details of the authorized Indian agent.
Steps to register CT scan in India
- Step 1: Applicant needs to determine if the device falls under the category of notified CT SCAN medical devices, which needs registration in India as per the notification of CDSCO.
- Step 2: Applicant needs to classify the CT SCAN devices in a risk-based class (Class C,) based on the risk they have
- Step 3: Applicant must fill out the application to get permission to manufacture based on the class Form M-7 (Class C & D) or Form MD- 8 (Loan license Class C) or Form MD-14 to import the CT SCAN medical device to market it in India.
- Step 4: The importer needs to appoint the Indian authorized agent, per the MDR,2017 rules, as the manufacturer’s legal representative who will look out for all the manufacturer’s official work if the manufacturer is not a residCT scan of India.
- STEP 5: Applicant needs to submit the following documents for the CT SCAN medical device:
- STEP 6: Notified body audits the manufacturing site within 60 days of application submission for Class C. Notified body sends the audit report to the Central licensing authority (CLA) within 30 days to permit the manuafcturing license whereas for import license no audit takes place. For import license audit report is needed, also if the need may be CLA can do the audit and the expense will be paid by the importer
- STEP 7: Central licensing authority (CLA) permits the license to manufacture CT SCAN medical devices through Form MD-9 (Class C) and Form MD-15 to import and Form MD -10 (Class C & D loan license to manufacturer).
- STEP 8: Market CT SCAN medical devices in India when it gets the green signal from the CDSCO.
TAT (Time for getting File approved):
3-4 Months After application submission.
Conclusion:The CT scan market is stood at USD203.32 million dollars and is expected to grow at 4.85 during the forecast year. Due to the advancement of technologies, there are many new products in the market, which is the main reason behind the strictness of rules. Since CT scan falls under Class C medical devices, their registration is mandatory. After the CDSCO notification, manufacturers and importers must register their CT scan machines before entering the Indian market. CDSCO aims to make the devices used safer and more effective. Thus, CDSCO is amending the laws to ensure that CT scan present in the market are safe, effective, and have a high benefit-risk ratio. Since they belong to moderate class risk, the manufacturer and importer need to pay special attention to their documents if they don’t want to interrupt the supply of their CT scan machines. Thus, it is advisable to consult an experienced regulatory person who can help to get the license without any delays.
Download Checklist of Documents Required for Registration of Medical Devices with CDSCO
These comprehensive set of checklists are designed to help streamline the registration process and ensure that you have all the necessary documentation in place.