1. Introduction
The organization in charge of creating federal regulations and outlining their specifications, as well as managing and overseeing the personal care industry or Cosmetic Product Registration in Dubai(UAE), is the Emirates Authority for Standardization and Metrology (ESMA). The Municipalities oversee inspections and market surveillance (one for each Emirate). The GSO 1943/2016 (Cosmetic Products – Safety Requirements of Cosmetic and Personal Care Products) contains health and safety regulations that must be followed when manufacturing cosmetics and personal care items. All cosmetics supplied or sold in the United Arab Emirates are subject to the UAE Cosmetics Regulation.
As per the Regulation, a cosmetic product is described as “any substance or mixture made to clean or perfume them, changing their appearance or enhancing their smell, or protecting or keeping them in the best shape” and used on the skin, hair, nails, lips, teeth, genitalia, or mucosa of the oral cavity. The Cosmetics Law does not apply to medical products (items used to treat illnesses) or tools and devices used with cosmetics.
ESMA has implemented the Emirates Conformity Assessment Scheme (ECAS) as a product certification program for all imported and locally made cosmetic and personal care products. ECAS ensures that goods (imported or locally made) meet all relevant technical requirements before being released onto the UAE market (health, packaging, labeling, and metrology). ESMA issues an ECAS Certificate of Conformity if the cosmetic product complies. A product can only be distributed or entered the nation with this certificate. After obtaining the certificate, cosmetic products must be registered with ESMA and the relevant Municipality (where the product is intended for sale). If the product can be sold in a chain of stores thanks to the Municipality registration, it can also be imported into the nation at the port of entry thanks to the ESMA registration.
Cosmetic Product Registration in Dubai, UAE can only be completed by a company incorporated in the United Arab Emirates (e.g., a local importer, manufacturer, or distributor).
Consequently, to register products in the United Arab Emirates, a local representative is required, as the company must be registered locally and hold a license for “general trading.” The Consumer Products Safety Section (CPSS) body can gather all the data required to assess a cosmetic product’s safety through registration. In the United Arab Emirates, a cosmetic product can only be produced, imported, exported, promoted, or distributed if it is legally compliant and registered.
2. The advantages of registering cosmetics
The registration of cosmetic products in Dubai UAE has numerous advantages. Among them are:
- It provides the flexibility to import and export while safeguarding the label.
- It protects the company’s reputation.
- It allows one to enter and exit Dubai or the United Arab Emirates with ease.
- Registering a product increases its value and keeps the business profitable.
3. The objectives of a cosmetic item
- To maintain in shape
- To alter appearance
- To eliminate body odor
- To tidy
- To shield
- To smell
4.Categories of Product

5. List of cosmetic items
The list of cosmetic products below is not all-inclusive; instead, it serves as an example of the kinds of products that may need to be registered in Dubai:
- Lotions, creams, gels, oils, and emulsions for the skin (face, hands, hands, feet, etc.)
- Shower and bath preparations
- Antiperspirant
- Depilatories
- Hair-care products:
- Product for waving, straightening, and fixing.
- Hair tints and bleaches
- Setting products, cleansing products, Conditioning products, Hairdressing products
- Tinted bases (liquids, pastes, powders)
- Face masks (except for peeling )
- Products for care of the teeth and mouth
- Products for external intimate hygiene
- Anti-wrinkle products.
- Sunbathing products
- Skin-whitening products
- Products for tanning without sun
- Makeup powders, after-bath powders, hygienic powders, etc.
- Perfumes, toilet water, and Eau de Cologne
- Toilet soaps, deodorant soaps, etc.
- Products for making up and removing makeup from the face and eyes.
- Shaving products (creams, foams, lotions)
- Products intended for application to the lips.
- Products for nail care and makeup
Exception: The product’s ingredients must be listed on the container or directly on the product in the absence of a container. The ingredients list (on the label of container packages) must contain allergens. A perfume’s list of ingredients (found on the container packaging) must state whether an aromatic allergenic substance is present in the product at a concentration greater than 0.001%. Except for four specific ingredients (which are included in the restricted list): alpha-hydroxy acids; urea; vitamin A and its esters (retinyl acetate, retinyl palmitate, and cetylpyridinium chloride); and urea, the UAE regulation’s positive, negative, and restricted lists of ingredients are generally in line with the lists of Annexes of the EU Cosmetics Regulation (Regulation (EC) No 1223/2009).
Other regulations that cosmetic products must adhere to in the United Arab Emirates(UAE) include being free of pork and all its derivatives, passing all safety tests mandated by law, and being safe when used in expected and typical circumstances. Cosmetics sold in the United Arab Emirates are prohibited from using designs, pictures, or words that contradict Islamic customs and the GCC’s (Gulf Cooperation Council) dominant social values.
6. How to register makeup products with the Municipality of Dubai
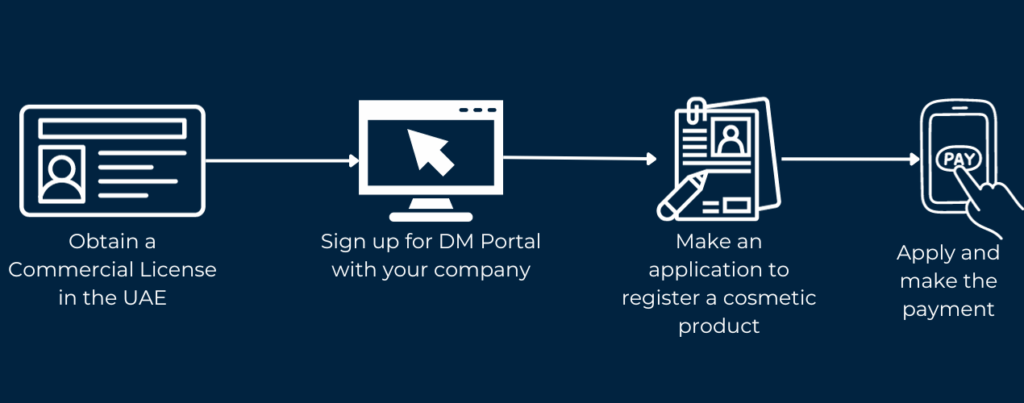
Your Step-by-Step Guide - Download Now
Download our simplified step-by-step guide to understand the Cosmetic Product Registration process in Dubai UAE. Streamline your licensing journey and ensure compliance with ease.
7. Documents Required in Cosmetic Product Registration in Dubai UAE:
- Clear Picture of Product.
- Clear Artwork of Product.
- Free Sale Certificate.
- Ingredients Report. (list of a product’s ingredients)
- Certificate of Analysis.
- GMP Certificate.
- If any other supporting documents (e.g. Halal Certificate, Organic Certificate, etc.)
- Trade License Copy (Local Company/Distributor)
- DM/Montaji Username and Password (For Local Company/Distributor)
Additional Documents Needed for Cosmetics Product Registration in Dubai UAE:
- Certificate of Free Sales
- Report on analysis
- Report from the laboratory
- Free Sales Certificate (CFS): A document proving that a product is freely sold in its country of origin is called a Certificate of Free Sales. The certificate, issued by the nation’s healthcare facilities or governing bodies, should originate from the government where the business was founded. The Certificate of Free Sales, or CFS, must, more precisely, be current when submitted.
- Analysis Report: The cosmetic product manufacturing company must provide an analysis report. The elements, chemical composition, and physical characteristics of the products used in the product’s manufacturing are all covered in detail in this document.
- A laboratory test report: This document lists the concentrations of specific metals, such as lead, cadmium, mercury, arsenic, and zinc. Additionally, it gives information about the required microbiology test, which must be performed in the country of origin or at Dubai Central Laboratory and is certified by both.
Fees: The cost of registering a product with the Dubai Municipality may vary depending on the product type. For instance, AED 230 (Govt Fee) is required to report cosmetic products.
Time Period: It takes about 22 working days for the cosmetics to process the application and receive the registration certificate after we have received the complete list of required documents and information for DM Product Registration. It will require an extra seven working days if the product needs to be tested in a lab. The authority of Dubai Municipality has the final say over the product registration approval. Within the DM processing system, there may be delays and changes to the processes.
8.Registration Requirements for Cosmetic Products on Labels
Product packaging must always have a product label. Generally, the label’s content must be written in English. It can be multilingual or bilingual, but each language’s content should be identical.
The label’s minimum requirements are:
- Brand Name
- Product Name (a summarized description of the product).
- Production & and expiration dates OR Period After Opening
- Manufacturer details
- Net weight or volume.
- The country of Origin should be declared clearly and should be specific.
- Product’s barcode.
- Batch number.
- Ingredients list
- Instructions for using the product
- Medical claims not allowed
Conclusion: Registering cosmetic products in Dubai UAE involves navigating a comprehensive framework governed by the Emirates Authority for Standardization and Metrology (ESMA) and the Dubai Municipality. Compliance with safety regulations, obtaining an ECAS Certificate of Conformity, and securing approvals from ESMA and the relevant Municipality are critical steps for market entry. The advantages of registration extend beyond mere compliance, offering flexibility in import-export activities, safeguarding reputations, and ensuring smooth business operations within Dubai and the United Arab Emirates. The meticulous documentation, testing, and labeling requirements underscore the commitment to consumer safety and satisfaction. As companies embark on this regulatory journey, understanding the nuances of the process, including costs and timelines, becomes pivotal for a successful and sustainable presence in Dubai’s dynamic cosmetic industry.







