Central Drug Standard Control Organization (CDSCO) is the national regulatory authority that regulates medical device sale, import, manufacture, stock, or distribution. Since medical devices have a direct impact on the health of the population, thus need keen observation. Not only manufacture or import but other activities such as labeling, packaging, and instruction of use are regulated by the regulatory body so that the medical device entities share no miscommunication or misinformation. Central Drug Standard Control Organization follows the Medical Device Rule, 2017; Drug and Cosmetic Act 1940; and Rule 1945 on what rules and regulations a person must keep in mind while selling medical devices in the Indian market. CDSCO involves obtaining a license to manufacture or import the medical device, proving that the person complies with rules and regulations set by the CDSCO and that the medical device sold is good quality, safe, and has no risks. Not only license to manufacture or import, but CDSCO looks at clinical evaluation and testing as well.
What is Medical Device?
As per Medical devices Rules, 2017 medical devices are defined as instruments, apparatus, appliances, implants, materials, etc., which can be used alone or in combination, such as software, or accessory, that are intended to be used for humans or animals, which helps in
- Diagnosing, preventing, monitoring, treating, or mitigating any disease, disorder, condition, injury, or disability
- Examination, replacement, alteration, or support of the anatomy or physiological process
- Supporting or sustaining the life
- Medical device disinfection
- Conception control
Classification of medical devices:
Medical device rule 2017 classifies the medical device into four classes depending on the risks involved, contact with the body, etc., namely:
Risk Class | Risk Involved |
Class A | Low risk |
Class B | Low-moderate risk |
Class C | Moderate high risk |
Class D | High Risk |
Stethoscope – A Stethoscope is an auscultation medical device that means medical device that helps to listen to the internal body sound, specifically the heart and lungs of the human or animal. It consists of a small hand-held with a curved tubing piece called a chest piece placed on the patient’s skin. The chest piece consists of a diaphragm and a bell on either side that amplifies the sounds depending on the condition being listened to. It also has an earpiece the doctor places in their ears, allowing them to listen to the amplified sound. Doctors and nurses commonly use stethoscopes.
Weight scales and weight scales with body composition – It is a medical device used to measure weight along with fat percentage. It is mainly used to treat patients who are overweight and suffers from obesity. It uses sensors present under the feet that use bioelectrical impedance.
Class of Mentioned devices – These devices are placed on the skin, which means it has direct contact with upper skin and is non-invasive, thus having no risks, and are placed in Class A no-risk device.
Forms required for Class A notified medical devices:
FORMS | DESCRIPTION | FEES |
Form MD-3 | Application form to manufacture Class A notified medical devices in India |
For one site INR 5000 and INR 500 for a medical device. |
Form MD-4 | Application form for loan license for Class A notified medical devices in India | |
Form MD-5 | Permission to manufacture Class A notified medical devices in India. | |
Form MD-6 | Permission for loan license to manufacture Class A notified medical devices in India. | |
Form MD-14 | Application to import Class A notified medical devices in India. |
$1000 for one site and $50 for one medical device unit |
Form MD-15 | Permission to import Class A notified medical devices into India. |
Steps to manufacture or import class a notified medical devices in india.
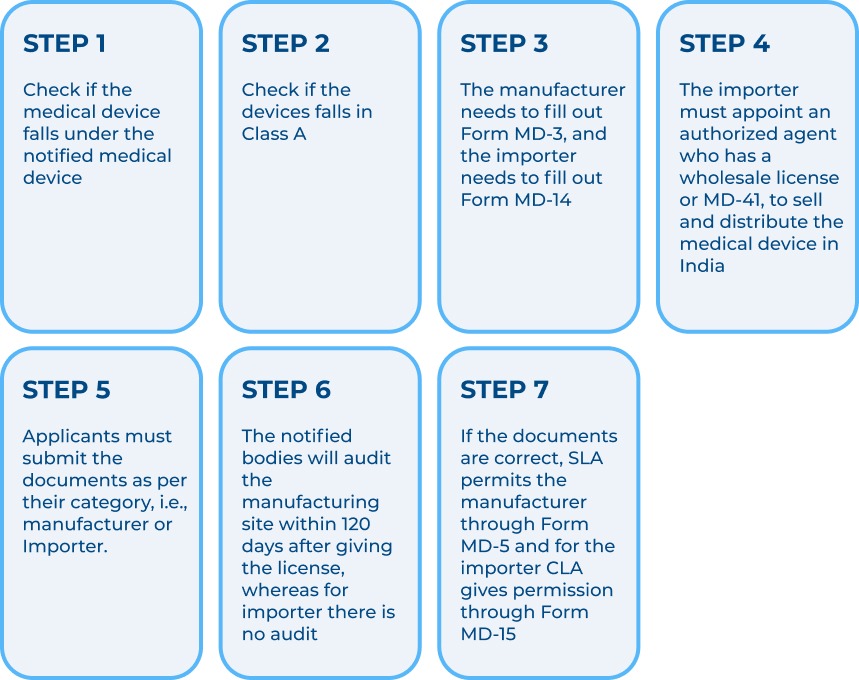
Documents for Manufacturers of Class A notified medical devices:
- Cover letter
- Fees
- Challan
- Constitution details of the manufacturing firm or authorized agent
- The Establishment /Site ownership /Tenancy Agreement.
- Copy of Duly notarized valid copies of Quality Certificate in respect to manufacturing site(s), if any;
- Copy of Certificate supporting quality management system (ISO: 13485), if any;
- Plant master file
- Device master file
- Test license (for the domestic manufacturer)
- Undertaking that the manufacturing site complies with the quality management system.
For the import license authorized agent needs to submit the following few more documents along with the documents mentioned above:
- Cover letter
- Power of attorney
- Wholesale license
- Free sale certificate from country of origin
- Free sale certificate from any country, namely USA, Australia, Canada, Japan, UK, and European Union countries.
- Inspection or audit report
- Certificates (ISO, full quality certificate, CE design certificate, Declaration of conformity)
- Plant master file (as per appendix I of MDR, 2017)
- Device master file (as per appendix II of MDR, 2017)
- Label and IFU
- Establishment certificate
- Constitution details of the authorized Indian agent.
Conclusion: CDSCO regulates the device and thus makes rules and regulations and follows a defined path that the applicant needs to follow if they want to sell their device. If there is any gap, the application can get rejected, penalized, or fined, causing financial loss and brand name loss. So, if the applicant wants to save time and hassle, then it is advisable to go through the document checklist, the steps to fill out the form, number of days it takes to get the application accepted so that the applicant can plan their product launch accordingly.
Download Checklist of Documents Required for Registration of Medical Devices with CDSCO
These comprehensive set of checklists are designed to help streamline the registration process and ensure that you have all the necessary documentation in place.







