Overview of CDSCO
Control Drug Standard Central Organization (CDSCO) is the regulatory body of India established by the Ministry of Health and Family Welfare (MoHFW). The main objective of CDSCO is to ensure the safety and effectiveness of the device being sold in the market. CDSCO follows the Medical Device Regulation,2017 and Drug and Cosmetic Act 1940, and Rules 1945 as reference guidelines. Any manufacturer or importer planning to sell and distribute their devices in India should know these guidelines. CDSCO is amending the laws to meet the ever-increasing demand for the devices. That’s why on 16th March 2022, The DCGI issued a notification related to CDSCO Registration for General Hospital Medical Devices, titled- “Classification of Medical Devices Pertaining to General Hospital,” governed by the Medical Device Rules, 2017.
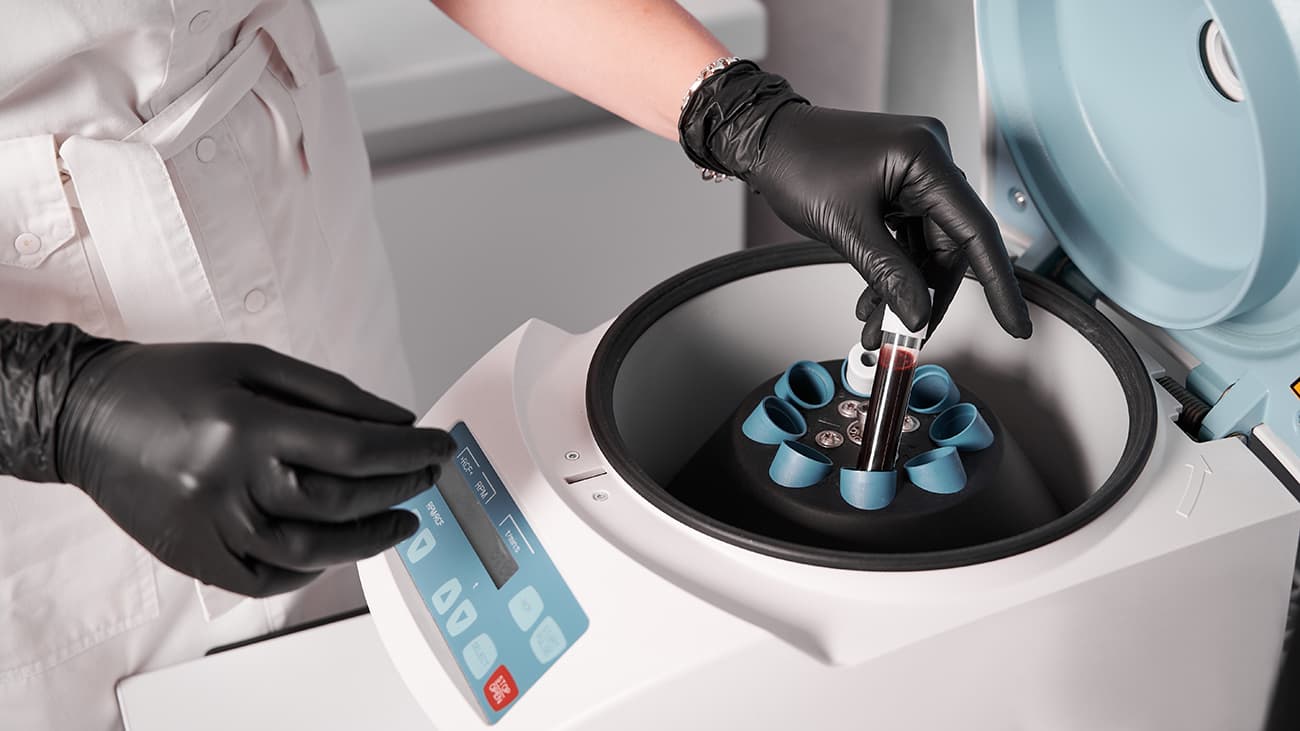
What is the centrifuge machine
A centrifuge can be defined as a machine that spins a tube or other container that consists of liquid at high speed, creating a strong force to separate the materials based on their density. Due to rotation, the heaviest components settle at the bottom, and lighter components remain suspended in the liquid.
Centrifuge can be used in a variety of clinical applications. One of them is the separation of blood components in blood samples. This process is used when researchers or any healthcare professional needs to separate the different components, such as white blood cells. Platelets etc., based on their density, size, and weight.
Type of centrifuge
There are few types of blood centrifuge –
- Small Benchtop Centrifuge: It is relatively small and straightforward, with a 3000 rpm top speed. It is typically used to collect quickly sedimenting materials like blood cells, yeast cells, or large precipitates of chemical reactions, which are common in the clinical laboratory for separating blood for plasma or serum, urine, and bodily fluids. It has no temperature regulation system. Depending on the diameter, approximately 100 tubes may be required.
- Microcentrifuge: Microcentrifuges can hold tubes with a small volume (up to 2 ml, or microcentrifuge tubes), or microfuges or Eppendorf centrifuges. It is particularly prevalent in biochemistry, molecular biology, microbiology, immunology/serology, blood banking, laboratory medicine, etc., areas of medical laboratories. It has a maximum force generation capacity of 15,000 x g, with or without refrigeration.
- High-Speed centrifuges: Centrifugal forces of 90000 g are produced at a maximum speed of 25000 rpm. A refrigerator was installed to reduce heat production. The thermocouple was used to keep the temperature between 0 and 4 °C. It can isolate sub-cellular organelles such as nuclei, mitochondria, and lysosomes and collect microbes, cell debris, cells, giant cellular organelles, and residues of chemical processes.
- Ultra-centrifuge and Miscellaneous Types of Centrifuges are a few more types of centrifuges used in clinical research and laboratory.
Class of Centrifuge
Centrifuge belongs to Class A non-measuring non-sterile medical devices and neither measures nor requires sterilization. All types of centrifuge fall under the Class A non-measuring non-sterile medical devices.
Registration Process of the Centrifuge in India
The registration process to manufacture or import Centrifuge (Class A non-sterile non-measuring) medical devices in India –Centrifuge are exempted from the requirement of manufacturing and import license (Form MD-15); however, other document requirements remain the same for anyone who wants to deal in hospital beds.
Step 1: Classify if the centrifuge falls under Class A nonsterile non-measuring medical device.
Step 2: If yes, the applicant must register their centrifuge from the “add non-regulatory device” section in the SUGAM portal.
Step 3: The applicant must select the purpose, i.e., whether they are the importer or the manufacturer.
Step 4: The applicant must upload the documents per their category.
Step 5: After uploading the documents, a “Registration number” will be generated that needs to be added to the label for sale in the Indian market.
Documents required for Centrifuge registration in India
For the manufacturer of hospital bed | For importers of hospital bed |
Name and address of the manufacturer of the hospital beds. | Name and address of the importer and manufacturing site where centrifuge are manufactured. |
Details of Class A medical device that comes under the non-sterile and non-measuring Class | Details of Class A medical device that comes under the non-sterile and non-measuring Class |
An undertaking from the manufacturer confirming that the centrifuge belongs to a Class A non-sterile and non-measuring medical device. | An undertaking from the importer confirming that the centrifuge belongs to a Class A non-sterile and non-measuring medical device. |
The self-certificate from the manufacturer to confirm that the product complies with the essential principles and standards specified in rule GSR 777. | The self-certificate from the importer to confirm that the product complies with the essential principles and standards specified in rule GSR 777. |
An undertaking from manufacturing stating that the details mentioned by the manufacturer are authentic. | Self-attested copy of the manufacturing site situated overseas in the origin country issued by the competent authority or Free Sale Certificate issued by the National Regulatory Authority. |
| An undertaking from the importer stating that the details mentioned by the manufacturer are authentic |
An undertaking from the importer stating that the details mentioned by the manufacturer are authentic
Note: No audit of the manufacturing site shall be necessary before granting a license or loan license to manufacture for sale or distribution of Class A medical device.
The applicant shall maintain the manufacturing or import records and their sale and distribution. When requested by the licensing authority, the applicant must provide the records for verification failure, which can lead to the cancellation of the license to sell centrifuge (Class A non-sterile non-measuring medical devices).
Conclusion: Centrifuge in Class A Non-sterile, non-measuring medical devices will require registration, but the documentation has been reduced. CDSCO is the regulatory body of India that regulates the medical device rules and regulations and gives registration licenses to deserving applicants. The main aim of the CDSCO is to make medical devices as safe as possible for the intended population, as it directly impacts people’s health.
Download Checklist of Documents Required for Registration of Medical Devices with CDSCO
These comprehensive set of checklists are designed to help streamline the registration process and ensure that you have all the necessary documentation in place.